Prints from color negatives are usually done by a large central lab that handles printing and processing for many local drug stores and supermarkets, or they may be done in-house using a mini-lab. The mini-lab is set up to do one roll of film at a time, whereas the product houses splice many rolls together and handle a high volume of pictures on a semi-continuous basis. In either case, the steps are the same as already discussed for generating a black-and-white negative image. The major difference comes in the printing process, where long rolls of color paper are pre-loaded into a printer. The roll of negatives is loaded, and the printer operator works in normal lights to preview each negative and make adjustments to the color balance. The color balance is adjusted by adding subtractive color filters to make the print more pleasing, particularly when it has been exposed incorrectly. There is only so much correction that can be done, so don't expect miracles. Once a full roll of paper is exposed, or a single roll of film has been printed (in the case of a mini-lab), the paper is processed.
Here are the steps in developing the color print paper after it is exposed:
- The latent-image sites are developed, and oxidized developer molecules combine with the color-forming couplers to create a silver image and a dye image. The reaction is stopped by a washing step.
- The silver image and any remaining unexposed silver halide is removed in a combined bleach-plus-fix solution (called the BLIX).
- The print is then carefully washed to remove any residual chemicals.
- The print is dried.
Once again, the gelatin binder swells to allow the processing chemicals access to the silver-halide grains, and allows fresh water to rinse out the by-products. The colored image should contain no residual silver.
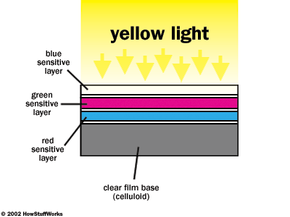
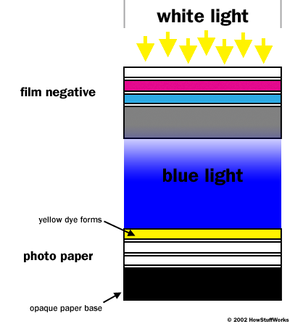
As a final example of color printing process, let's take a look at our negative that was exposed to a pure yellow object. When the resultant negative is placed in the printer, and white light is shown through the negative onto the color paper, here is what happens. The white light exposure is the equivalent of a color print exposure. Only blue light gets through the color negative and exposes the color paper. The exposed color paper then forms yellow dye in the blue-sensitive layer, and the original color is reproduced.
If you've made it this far, you are to be congratulated! Photography isn't as easy as it seems, but then again, that is what makes it so remarkable. The ability to capture and record individual photons of light and turn them into a lasting memory requires many steps. If any one of them goes wrong, the entire result may be lost. On the other hand, when all the stuff works, the results are truly astounding.
For more information on photographic film and related topics, check out the links on the next page.