Light and Energy
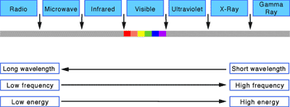
Energy from the sun comes to the Earth in visible and invisible portions of the electromagnetic spectrum. Human eyes are sensitive to a small portion of that spectrum that includes the visible colors -- from the longest visible wavelengths of light (red) to the shortest wavelengths (blue).
Microwaves, radio waves, infrared, and ultraviolet waves are portions of the invisible electromagnetic spectrum. We cannot see these portions of the spectrum with our eyes, but we have invented devices (radios, infrared detectors, ultraviolet dyes, etc.) that let us detect these portions as well.
Advertisement
Light is neither a wave nor a particle, but has properties of both. Light can be focused like a wave, but its energy is distributed in discrete packets called photons. The energy of each photon is inversely related to the wavelength of the light -- blue light is the most energetic, while red light has the least energy per photon of exposure. Ultraviolet light (UV) is more energetic, but invisible to human eyes. Infrared light is also invisible, but if it is strong enough our skin detects it as heat.
It is the energy in each photon of light that causes a chemical change to the photographic detectors that are coated on the film. The process whereby electromagnetic energy causes chemical changes to matter is known as photochemistry. By carefully engineering materials, they can be chemically stable until they are exposed to radiation (light). Photochemistry comes in many different forms. For example, specially formulated plastics can be hardened (cured) by exposure to ultraviolet light, but exposure to visible light has no effect. When you get a sun tan, a photochemical reaction has caused the pigments in your skin to darken. Ultraviolet rays are particularly harmful to your skin because they are so energetic.