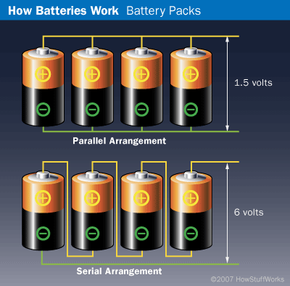
Battery Arrangement and Power
In many devices that use batteries -- such as portable radios and flashlights -- you don't use just one cell at a time. You normally group them together in a serial arrangement to increase the voltage or in a parallel arrangement to increase current. The diagram shows these two arrangements.
The upper diagram shows a parallel arrangement. The four batteries in parallel will together produce the voltage of one cell, but the current they supply will be four times that of a single cell. Current is the rate at which electric charge passes through a circuit, and is measured in amperes. Batteries are rated in amp-hours, or, in the case of smaller household batteries, milliamp-hours (mAH). A typical household cell rated at 500 milliamp-hours should be able to supply 500 milliamps of current to the load for one hour. You can slice and dice the milliamp-hour rating in lots of different ways. A 500 milliamp-hour battery could also produce 5 milliamps for 100 hours, 10 milliamps for 50 hours, or, theoretically, 1,000 milliamps for 30 minutes. Generally speaking, batteries with higher amp-hour ratings have greater capacities.
Advertisement
The lower diagram depicts a serial arrangement. The four batteries in series will together produce the current of one cell, but the voltage they supply will be four times that of a single cell. Voltage is a measure of energy per unit charge and is measured in volts. In a battery, voltage determines how strongly electrons are pushed through a circuit, much like pressure determines how strongly water is pushed through a hose. Most AAA, AA, C and D batteries are around 1.5 volts.
Imagine the batteries shown in the diagram are rated at 1.5 volts and 500 milliamp-hours. The four batteries in parallel arrangement will produce 1.5 volts at 2,000 milliamp-hours. The four batteries arranged in a series will produce 6 volts at 500 milliamp-hours.
Battery technology has advanced dramatically since the days of the Voltaic pile. These developments are clearly reflected in our fast-paced, portable world, which is more dependent than ever on the portable power source that batteries provide. One can only imagine what the next generation of smaller, more powerful and longer-lasting batteries will bring.
For more information on batteries and related topics, check out the links below.
Battery FAQ
What is battery energy?
What are the different types of batteries?
How much is a car battery?
What is the energy source of a battery?
What type are rechargeable batteries?
Related Articles
More Great Links
- BBC: How Do Batteries Work?
- The Official Lemon-Power Website (It's about using lemons to power stuff!)
- Georgia Tech Center for Fuel Cell and Battery Technologies
Sources
- American Chemical Society. "History of the Battery." National Historic Chemical Landmarks. 2005. (June 23, 2011) http://acswebcontent.acs.org/landmarks/drycell/history.html
- "Batteries." Intro to Physical Computing, New York University. April 19, 2011. (June 23, 2011) http://itp.nyu.edu/physcomp/Notes/Batteries
- Brand, Mike, Shannon Neaves, and Emily Smith. "Museum of Electricity and Magnetism." National High Magnetic Field Laboratory. 2011. (June 25, 2011) http://www.magnet.fsu.edu/education/tutorials/museum/index.html
- Buckle, Kenneth. "How Do Batteries Store and Discharge Electricity?" Scientific American. May 29, 2006. (June 23, 2011) http://www.scientificamerican.com/article.cfm?id=how-do-batteries-store-an
- CalRecycle. "Rechargeable Batteries and Chargers: A Personal Perspective." Sept. 9, 2009. (June 25, 2011) http://www.calrecycle.ca.gov/ReduceWaste/power/rechbattinfo.htm
- California Energy Commission. "Lemon Power." 2006. (June 22, 2011) http://www.energyquest.ca.gov/projects/lemon.html
- Coyne, Kristen Eliza. "Interactive Tutorials." National High Magnetic Field Laboratory. 2011. (June 23, 2011) http://www.magnet.fsu.edu/education/tutorials/java/index.html
- Davidson, Michael W. "Electricity and Magnetism: Batteries." Jan. 28, 2003. (June 22, 2011) http://micro.magnet.fsu.edu/electromag/electricity/batteries/index.html
- Decker, Franco. "Volta and the 'Pile.'" Electrochemistry Encyclopedia. January 2005. (June 23, 2011) http://electrochem.cwru.edu/encycl/art-v01-volta.htm
- Duracell. "Power Education." 2010. (June 23, 2011) http://www.duracell.com.au/en-AU/power-education/index.jspx
- Energizer. "Learning Center." 2011. (June 22, 2011) http://www.energizer.com/learning-center/Pages/facts-history-care.aspx
- Environmental Protection Agency. "Batteries." Dec. 1, 2010. (June 22, 2011) http://www.epa.gov/osw/conserve/materials/battery.htm
- Frood, Arran. "Riddle of 'Baghdad's Batteries.'" BBC News. Feb. 27, 2003. (June 23, 2011) http://news.bbc.co.uk/2/hi/science/nature/2804257.stm
- GreenBatteries. "Information on Environmentally Friendly Rechargeable Batteries." 2011. (June 25, 2011) http://www.greenbatteries.com/faqs.html
- Idaho Public Television. "Electricity Facts." 2011. (June 25, 2011) http://idahoptv.org/dialogue4kids/season6/electricity/facts.cfm
- Iggulden, Hal. "The Dangerous Book for Boys." New York: HarperCollins Publishers, Inc., 2007.
- Komando, Kim. "Learn How to Maximize Battery Performance." USA Today. Aug. 7, 2005. (June 25, 2011) http://www.usatoday.com/tech/columnist/kimkomando/2005-08-07-battery-life_x.htm
- Manjoo, Farhad. "Better Batteries Will Save the World." Slate. June 21, 2011. (June 23, 2011) http://www.slate.com/id/2297125/
- Rahim, Saqib. "Will Lithium-Air Battery Rescue Electric Car Drivers from 'Range Anxiety?'" The New York Times. May 7, 2010. (June 22, 2011) http://www.nytimes.com/cwire/2010/05/07/07climatewire-will-lithium-air-battery-rescue-electric-car-37498.html?pagewanted=1
- Savage, Neil. "Batteries That Breathe." DiscoveryNews. Feb. 8, 2011. (June 22, 2011) http://news.discovery.com/tech/batteries-that-breathe-110208.html
- University of Hawaii HAM Club. "Batteries in Fact and Fiction." August 1999. (June 22, 2011) http://www.chem.hawaii.edu/uham/bat.html