Explaining the Voltaic Pile
A voltaic pile is an early form of electric battery. Italian physicist Alessandro Volta stacked piles of alternating metal copper and zinc discs separated by pieces of cloth or cardboard soaked in an electrolyte solution. When the metals and the electrolyte come into contact, a chemical reaction occurs, generating an electrical potential difference between the metal layers.
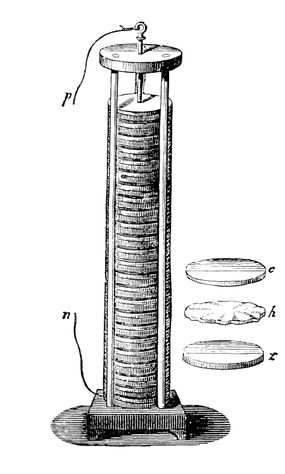
This potential difference allows for the flow of electric current through an external circuit connected to the pile. Volta’s piles served as a precursor to modern electrical batteries and played a crucial role in shaping the development of electrical technology and our understanding of electricity.
Advertisement
Luigi Galvani's Influence on Alessandro Volta
By the time Luigi Galvani started his own electrical experiments, other scientists and inventors had already delved into this area. Galvani, an Italian physician, conducted experiments that led him to further study animal electricity. He observed:
Like others, Volta believed Galvani’s theory. However, he also wanted to learn more about the connection between the metal plates and the strength of the contractions.
By 1792, about a year after Galvani’s statements, Volta disagreed with Galvani, discussing metallic electricity in publications and letters. He would later do more experiments with animal electricity. And by 1800, he had introduced the voltaic pile.
Introduction of Volta’s Piles
In the time that he spoke out against Galvani's theory and in the year 1800, there was plenty of discussion about the validity of animal electricity. In a letter to Sir Joseph Banks of the London Royal Society, he introduced the voltaic pile and discussed the electricity produced.
“The apparatus to which I allude, and which will, no doubt, astonish you, is only the assemblage of a number of good conductors of different kinds arranged in a certain matter,” he wrote. “Thirty, forty, sixty, or more pieces of copper, or rather silver, applied each to a piece of tin, or zinc, which is much better, and as many strata of water, or any other liquid which may be a better conductor, such as salt water, ley, &c., or pieces of pasteboard, skin, &c., well soaked in these liquids; such strata interposed between every pair or combination of two different metals in an alternate series, and always in the same order of these three kinds of conductors, are all that is necessary for constituting my new instrument…”
Volta’s Impact
Volta’s work contributed to the understanding of electric current and electromotive force.
He demonstrated the connection between chemical reactions and electricity, opening up new avenues of scientific exploration. English chemist William Nicholson, for example, created something similar to voltaic piles and began running experiments.
Nicholson along with Anthony Carlisle found that dipping the poles of the battery into water resulted in a chemical transformation spurred by electric energy. The discovery of water electrolysis complemented Volta’s discoveries.
Homemade Battery Experiments
If you want to learn more about the electrochemical reactions that occur in batteries, you can actually build one yourself using simple household materials.
One thing you should buy before you start is an inexpensive ($10 to $20) volt-ohm meter at your local electronics or hardware store. Make sure that the meter can read low voltages (in the one-volt range) and low currents (in the five-to-10 milliamp range). With this equipment on hand, you'll be able to see exactly how well your battery is performing.
You can create your own voltaic pile using quarters, foil, blotting paper, cider vinegar and salt.
- Cut the foil and blotting paper into circles.
- Soak the blotting paper in a mixture of the cider vinegar and salt.
- Using masking tape, attach a copper wire to one of the foil discs.
- Now stack the materials in this order: foil, paper, quarter, foil, paper, quarter, and so on until you have repeated the pattern 10 times.
- Once the last coin is on the stack, attach a wire to it with masking tape.
- Finally, attach the free ends of the two wires to an LED, which should light up.
In this experiment, the copper in the quarter is the cathode, the foil is the anode, the cider vinegar-salt solution is the electrolyte, and the blotting paper is the separator.
You can also create a homemade battery can also be made from copper wire, a paper clip and a lemon.
- First, cut a short piece of copper wire and straighten out the paper clip.
- Use sandpaper to smooth out any rough parts on the ends of either piece of metal.
- Next, gently squeeze the lemon by rolling it on a table, but be careful not to break the skin.
- Push the copper wire and the paper clip into the lemon, ensuring that they are as close together as possible without actually touching.
- Finally, connect your volt-ohm meter to the ends of the paper clip and the copper wire, and see what kind of voltage and current your battery produces.
By now you should be well-acquainted with the basic principles by which batteries discharge electricity. Read on to discover how some batteries can be recharged.
This page was updated in conjunction with AI technology, then fact-checked and edited by a HowStuffWorks editor.