Battery History
Batteries have been around longer than you may think. In 1938, archaeologist Wilhelm Konig discovered some peculiar clay pots while digging at Khujut Rabu, just outside of present-day Baghdad, Iraq. The jars, which measure approximately 5 inches (12.7 centimeters) long, contained an iron rod encased in copper and dated from about 200 B.C. Tests suggested that the vessels had once been filled with an acidic substance like vinegar or wine, leading Konig to believe that these vessels were ancient batteries. Since this discovery, scholars have produced replicas of the pots that are in fact capable of producing an electric charge. These "Baghdad batteries" may have been used for religious rituals, medicinal purposes, or even electroplating.
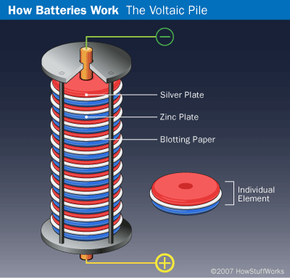
In 1799, Italian physicist Alessandro Volta created the first battery by stacking alternating layers of zinc, brine-soaked pasteboard or cloth, and silver. This arrangement, called a voltaic pile, was not the first device to create electricity, but it was the first to emit a steady, lasting current. However, there were some drawbacks to Volta's invention. The height at which the layers could be stacked was limited because the weight of the pile would squeeze the brine out of the pasteboard or cloth. The metal discs also tended to corrode quickly, shortening the life of the battery. Despite these shortcomings, the SI unit of electromotive force is now called a volt in honor of Volta's achievement.
Advertisement
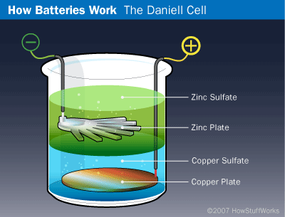
The next breakthrough in battery technology came in 1836 when English chemist John Frederick Daniell invented the Daniell cell. In this early battery, a copper plate was placed at the bottom of a glass jar and a copper sulfate solution was poured over the plate to half-fill the jar. Then the zinc plate was hung in the jar, and a zinc sulfate solution was added. Because copper sulfate is denser than zinc sulfate, the zinc solution floated to the top of the copper solution and surrounded the zinc plate. The wire connected to the zinc plate represented the negative terminal, while the one leading from the copper plate was the positive terminal. Obviously, this arrangement would not have functioned well in a flashlight, but for stationary applications it worked just fine. In fact, the Daniell cell was a common way to power doorbells and telephones before electrical generation was perfected.
By 1898, the Colombia Dry Cell became the first commercially available battery sold in the United States. The manufacturer, National Carbon Company, later became the Eveready Battery Company, which produces the Energizer brand.
Now that you know some of the history, click over to the next page to learn the various parts of a battery.