Lithium-ion batteries are incredibly popular these days. You can find them in laptops, PDAs, cell phones and iPods. They're so common because, pound for pound, they're some of the most energetic rechargeable batteries available.
Lithium-ion batteries have also been in the news lately. That’s because these batteries have the ability to burst into flames occasionally. It's not very common — just two or three battery packs per million have a problem — but when it happens, it's extreme. In some situations, the failure rate can rise, and when that happens you end up with a worldwide battery recall that can cost manufacturers millions of dollars.
Advertisement
So the question is, what makes these batteries so energetic and so popular? How do they burst into flame? And is there anything you can do to prevent the problem or help your batteries last longer? In this article, we'll answer these questions and more.
Lithium-ion batteries are popular because they have a number of important advantages over competing technologies:
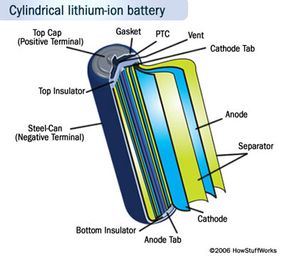
- They're generally much lighter than other types of rechargeable batteries of the same size. The electrodes of a lithium-ion battery are made of lightweight lithium and carbon. Lithium is also a highly reactive element, meaning that a lot of energy can be stored in its atomic bonds. This translates into a very high energy density for lithium-ion batteries. Here is a way to get a perspective on the energy density. A typical lithium-ion battery can store 150 watt-hours of electricity in 1 kilogram of battery. A NiMH (nickel-metal hydride) battery pack can store perhaps 100 watt-hours per kilogram, although 60 to 70 watt-hours might be more typical. A lead-acid battery can store only 25 watt-hours per kilogram. Using lead-acid technology, it takes 6 kilograms to store the same amount of energy that a 1 kilogram lithium-ion battery can handle. That's a huge difference [source: Everything2.com].
- They hold their charge. A lithium-ion battery pack loses only about 5 percent of its charge per month, compared to a 20 percent loss per month for NiMH batteries.
- They have no memory effect, which means that you do not have to completely discharge them before recharging, as with some other battery chemistries.
- Lithium-ion batteries can handle hundreds of charge/discharge cycles.
That is not to say that lithium-ion batteries are flawless. They have a few disadvantages as well:
- They start degrading as soon as they leave the factory. They will only last two or three years from the date of manufacture whether you use them or not.
- They are extremely sensitive to high temperatures. Heat causes lithium-ion battery packs to degrade much faster than they normally would.
- If you completely discharge a lithium-ion battery, it is ruined.
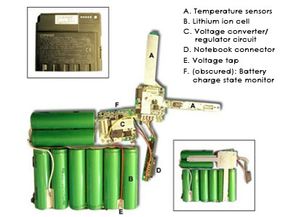
- A lithium-ion battery pack must have an on-board computer to manage the battery. This makes them even more expensive than they already are.
- There is a small chance that, if a lithium-ion battery pack fails, it will burst into flame.
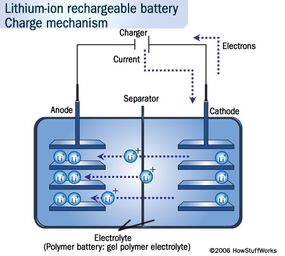
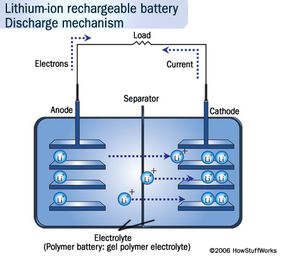
Many of these characteristics can be understood by looking at the chemistry inside a lithium-ion cell. We'll look at this next.
Advertisement