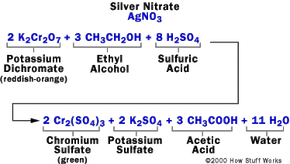
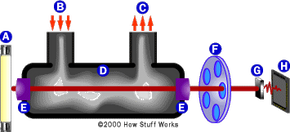
We hear and read about drivers involved in an accident who are later charged with drunken driving, and usually a news report on the accident will reveal the driver's blood alcohol content. A driver might be found to have a level of 0.15, for example, while the legal limit is 0.08. To comprehend how we can gain access to such precise numbers, we have to ask ourselves: how does a breathalyzer work?
What do those figures mean? And how do police officers find out if someone they suspect has been drinking is actually legally drunk? Many officers in the field rely on breath alcohol testing devices to determine the blood alcohol concentration (BAC) in drunken-driving suspects. In this article, we will examine the scientific principles and technology behind the Breathalyzer and other breath alcohol testing devices.
Advertisement