Light emitting diodes, commonly called LEDs, are real unsung heroes in the electronics world. They do many different jobs in all kinds of devices. They form numbers on digital clocks, transmit information from remote controls, light up watches and tell you when your appliances are turned on. Collected together, they can form images on a jumbo television screen or illuminate a traffic light.
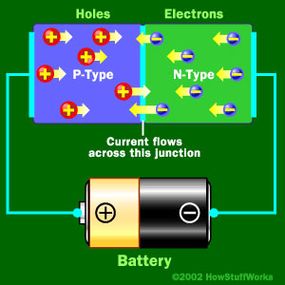
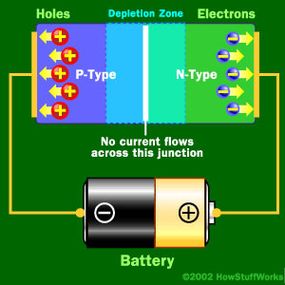
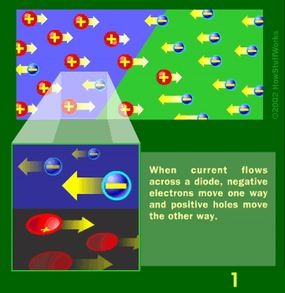
Basically, LEDs are just tiny light bulbs that fit easily into an electrical circuit. But unlike incandescent bulbs, they don't have filaments that burn out, they use less electricity, and they don't get especially hot. They're illuminated solely by the movement of electrons in a semiconductor material, and they last just as long as a standard transistor. The life span of an LED surpasses the short life of an incandescent bulb by thousands of hours. Because of these advantages, tiny LEDs are one of the most popular technologies used to light LCD TVs.
Advertisement
LEDs have several advantages over conventional incandescent lamps, but their main advantage is efficiency. In incandescent bulbs, the light-production process involves generating a lot of heat (the filament must be warmed to illuminate). This energy is completely wasted unless you're using the lamp as a heater, because a huge portion of the available electricity isn't going toward producing visible light. LEDs generate very little heat, relatively speaking. A much higher percentage of the electrical energy is going directly to generating light, which cuts down the electricity demands considerably.
Per watt, LEDs output more lumens (or quantities of visible light) than regular incandescent bulbs. Light emitting diodes have a higher luminous efficacy (how efficiently electricity is converted to visible light) than incandescents – a 60-watt incandescent bulb can generate between 750-900 lumens, but you can get the same output from a LED bulb using only 6-8 watts. And that same LED bulb can last 25,000 hours, but the 60-watt incandescent is only likely to light up for about 1,200 hours. In other words, one LED bulb can last as long as 21 60-watt incandescent bulbs burned consecutively [source: EarthEasy].
Until recently, LEDs were too expensive to use for most lighting applications because they're built around advanced semiconductor material. The price of semiconductor devices plummeted after the year 2000, however, making LEDs a more cost-effective lighting option for a wide range of situations. While they may be more expensive than incandescent lights up front (about $5 versus $1 for incandescent bulbs), their lower cost in the long run can make them a better buy. Several companies have begun selling LED light bulbs designed to compete with incandescent and compact fluorescents that promise to deliver long lives of bright light and amazing energy efficiency.
In this article, we'll examine the technology behind these ubiquitous blinkers, illuminating some cool principles of electricity and light in the process.
Advertisement