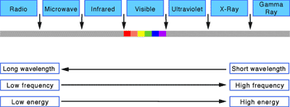
Light Frequencies
Once Maxwell introduced the concept of electromagnetic waves, everything clicked into place. Scientists now could develop a complete working model of light using terms and concepts, such as wavelength and frequency, based on the structure and function of waves. According to that model, light waves come in many sizes. The size of a wave is measured as its wavelength, which is the distance between any two corresponding points on successive waves, usually peak to peak or trough to trough. The wavelengths of the light we can see range from 400 to 700 nanometers (or billionths of a meter). But the full range of wavelengths included in the definition of electromagnetic radiation extends from 0.1 nanometers, as in gamma rays, to centimeters and meters, as in radio waves.
Light waves also come in many frequencies. The frequency is the number of waves that pass a point in space during any time interval, usually one second. We measure it in units of cycles (waves) per second, or hertz. The frequency of visible light is referred to as color, and ranges from 430 trillion hertz, seen as red, to 750 trillion hertz, seen as violet. Again, the full range of frequencies extends beyond the visible portion, from less than 3 billion hertz, as in radio waves, to greater than 3 billion billion hertz (3 x 1019), as in gamma rays.
Advertisement
The amount of energy in a light wave is proportionally related to its frequency: High frequency light has high energy; low frequency light has low energy. So, gamma rays have the most energy (part of what makes them so dangerous to humans), and radio waves have the least. Of visible light, violet has the most energy and red the least. The whole range of frequencies and energies, shown in the accompanying figure, is known as the electromagnetic spectrum. Note that the figure isn't drawn to scale and that visible light occupies only one-thousandth of a percent of the spectrum.
This might be the end of the discussion, except that Albert Einstein couldn't let speeding light waves lie. His work in the early 20th century resurrected the old idea that light, just maybe, was a particle after all.